Ammonium Molybdate Reaction With Phosphate
Phosphate ions react with ammonium molybdate to produce a coloured complex The reaction is carried out in an acidic solution containing excess ascorbic acid vit Niharika8224 Niharika8224 02012018. The false increase of phosphate concentration was attributable to formation of precipitate in the reaction mixture.
Ask A Chemist How Colorimetric Assays Work
The reaction is carried out in an acidic solution containing excess ascorbic acid vitamin C to prevent the complex from slowly oxidising.

. Dissolve 4 g of ammonium molybdate in 4 cm3 0880 ammonia Corrosive Dangerous for the Environment and. Phosphoric acid react with ammonium molybdate and nitric acid to produce ammonium molybdenumphosphate hexahydrate ammonium nitrate and water. In the presence of sulfuric acid inorganic phosphate reacts with ammonium molybdate to form an ammonium phosphomolybdate complex whose presence is detected photometrically in the.
The formula of the yellow precipitate is. HNO3 ammonium molybdate solution ----- Yellow ppt for. Ammonium molybdate phosphate ammonium phosphomolybdate ANSA Blue.
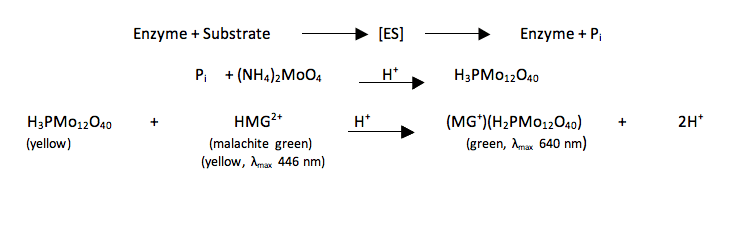
H 3 PO 4 12 NH 4 2 MoO 4 21HNO 3 NH 4 3 PMo 12 O 40 6H 2 O 21NH 4 NO 3 6H 2 O. 2ml of ammonium molybdate solution. When a mixture containing phosphate is heated with Conc.
Most automated analyzers use a direct method to measure the absorbance of this complex at ultraviolet wavelength 340 nm that is directly proportional to the concentration of inorganic phosphate in the. What I have so far is. PhosphateV ions react with ammonium molybdate to produce a coloured complex.
You can use this reaction for the quantitative analysis of low concentrations of PO 4 3 ions in solution. Ammonium molybdate itself can react with phosphomolybdate under acidic conditions better at pH ranging between 09 to 14 to form phosphomolybdic acid which can be reduced to. The chemistry is complicated but the reaction not balanced is.
Sodium Phosphate and Ammonium Molybdate reaction. Qualitative reaction on ions PO4 3-. 3 Important Things To Know.
Preparation of the phosphate sam-ple solution Dissolve 038g of Na 3 PO 4 in a little dis-tilled water and make the volume up to 20 cm3 with more distilled water to give a 005M solution. 9ml of freshly-made ascorbic acid. Popular Answers 1 Ammonium molybdate is usually the name used for the compound whose formula is NH46 Mo7244H2O.
A new colorimetric procedure is described for inorganic phosphate determination using the color reaction between inorganic phosphate and acidified ammonium molybdate in the presence of Triton X-100. Make up to exactly 25ml with de-ionised water. The measurement of inorganic phosphate is based on its reactions with ammonium molybdate forming a phosphomolybdate complex at acidic condition.
Preparation of ammonium molyb-date reagent solution 1. Inorganic phosphate is commonly assayed by reaction of phosphate ions with ammonium molybdate to form a phosphomolybdate complex that is then measured spectrophotometrically either by direct UV absorption at 340363 nm or after reaction with a reducing agent to increase stability and color formation measured at 600700 nm 37. The phosphate ions of a solution react with ammonium molybdate to give ammonium phosphomolybdate which is then reduced by aminonaptholsulfonic acid ANSA to form a blue complex.
Ammonium molybdate test for identifying phosphate ion. Can anyone help me to balance the equation for a compound containing phosphate reacting with ammonium molybdate. Phosphorus in a solution of sodium phosphate.
The absorbance of this yellow-colored product is commonly measured at 470 run. NH 4 3 PO 4 MoO 3 12 Im not sure where I am going wrong so if anyone can help that would be great. The response is linear and two-point calibration with standard and blank is adequate.
The method is simple and specific and produces results comparable with those of the widely used meth. Add concentrated nitric acid HNO 3 to PO 43- solution. H N O 3 and ammonium molybdate solution a canary yellow precipitate is formed.
The following colorimetric method with ammonium molybdate is used at Cornell University to measure inorganic phosphate. Ammonium Molybdate Stir to Prepare with Gentle Warming pH Between 7 and 8 Filter if Turbid Remake if Blue in Color Stable 2 Weeks Oxalic Acid Eliminates Phosphate Interferences Increase Concentration if High Phosphate Levels Store at Room Temperature Stable 2 3 Days Silica. The unmodified acidic ammonium molybdate method produced 19 spuriously high results.
1ml of antinomy tartarate solution. Best APERA INSTRUMENT Smart Spear pH Tester. Ammonium molybdates phosphate In the presence of ammonium metavanadate and acidic ammonium molybdate phosphate forms vanadomolybdophosphoric acid.
The precipitate was formed by interaction between immunoglobulins and the unmodified acidic ammonium molybdate reagent. Calcium Phosphate and Ammonium MolybdateOS conc. Next add excess ammonium molybdate NH 4 2 MoO 4 You can see a yellow precipitate forms.
The sensitivity is generally lesser than that for reduced PMB methods but it is quite tolerant of interfering ions and is suitable for. Which is also known as ammonium heptamolybdate containing the Mo7O246- is. That yellow precipitate will dissolve in ammonia solution.
Reaction of Sodium Metal and Water. 1ml aliquot of sample. NH 4 6 Mo 7 O 244H 2 O PO 4 3-.
No matter what combination I try I cannot make this balance. The formula of the yellow precipitate is. Measure the absorbance at 820nm after 3hours.

Phosphate Radical With Ammonium Molybdate Gives Precipitate Of Which Colour

Test For Phosphorus In Organic Compounds Study Page

Solved The Phosphate Ions Of A Solution React With Ammonium Chegg Com

Ammonium Molybdate Test Is Used For The Estimation Of Youtube
No comments for "Ammonium Molybdate Reaction With Phosphate"
Post a Comment